ORIGIN, EFFECTS AND TREATMENTS
The elimination of hydrogen sulfide (H2S) in biogas becomes a necessity not only to keep the pipelines, machines and equipment involved in its transportation, use and application in good condition but also, and above all, to preserve the life of all the personnel in charge of the operation and maintenance of the biogas production stations, as well as avoiding emissions of toxic gases into the atmosphere.
Gas-phase: Hydrogen sulfide (H2S)
Liquid phase: Hydrogen sulfide (H2S)
Detection: Strong odour of rotten eggs.
Origin.
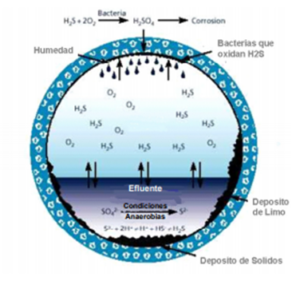
Hydrogen sulfide (H2S) is present in most putrefaction processes or biological degradation of organic matter when this process is carried out in the absence of oxygen. Under anaerobic conditions, sulfur-reducing bacteria acquire oxygen from sulfate (SO4) converting it into sulfide (S2-) that will give rise to H2S and hence the presence of H2S in biogas. Figure 1
The hydrogen sulfide concentration in the gas is a function of the digester feed substrate and the inorganic sulfate content. Wastes with a high protein content with sulfur-based amino acids (methionine and cysteine) can significantly influence the levels of hydrogen sulfide in the biogas, as well as the presence of sulfates in the water or liquids to be treated. The concentrations of H2S, therefore, vary according to the substrate to be treated and its production is generally linked to the organic load that said substrate presents.
Figure 1. Mechanism by which H2S is produced and how it corrodes a piping system.
Figure 1. Mechanism of corrosion and formation of H2S
Effects
The elimination/reduction of hydrogen sulfide (H 2 S) is necessary due to its polluting effect at different levels, among which can be mentioned.
- About the environment: acid rain production.
- On equipment and ducts: Corrosion and minimization of its useful life.
Corrosion caused by the presence of hydrogen sulfide in treatment plants is a well-studied and documented problem. This corrosion starts when bacteria oxidize H2S to sulfuric acid on pipe surfaces or concrete walls. Sulfuric acid corrodes concrete (such is the case of the concrete base on which biogas storage gasholders are installed), after which the steel frame is exposed and begins to corrode as well.
Figures 2 show the corrosion produced in different parts of the cogeneration system by the presence of H2S in the biogas.

Figures 2. Corrosion in the recuperator, silencer and piston due to the presence of H2S in the biogas.
- About people.
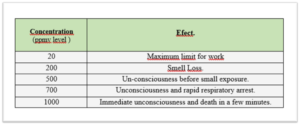
Table Nº 1 shows the effects that the concentration level produces on the human being who is in charge of the operation of the biogas installations.
One of the most important aspects in the cleaning of biogas in the different facilities that produce this gas is the elimination of the concentration of H2S before its storage or use/application due to the damage it produces in the different equipment and machines, as well as in the personnel in charge of the operation of these types of facilities.
TREATMENTS. H2S REMOVAL TECHNIQUES IN BIOGAS.
Currently, there are many processes available on the market for the removal of hydrogen sulfide (H2S) from biogas, each with its advantages and limitations. Some are limited by the level of H2S and/or CO2 in the gas stream. Others do not adequately remove mercaptans. And others are not suitable due to their complexity of operation, initial capital investment and/or high maintenance/operation costs.
In the case of WWTPs (wastewater treatment plants), there are two points or parts within the facility where this desulfurization process can be carried out.
- At origin, that is, in the reactor or pretreating the sludge to be digested. In the case of the digester, we can talk about the injection of air, the injection of oxygen in the dome or the addition of iron hydroxide or ferric chloride. In the case of sludge, thermal hydrolysis or the addition of iron hydroxide in the sludge flow entering the reactor is of interest.
- Online. The biogas that comes out of the digester once carries out the gross cleaning, that is, the elimination of foams, particles, condensates and sediments.
Desulfurization processes can be broadly classified into dry and wet. In turn, these can be divided into those in which there is a chemical reaction or those in which there is no chemical reaction.
1.- Dry desulfurization processes.
- Impregnated activated carbon (NaOH, KI, etc). Catalytic activated carbon.
- molecular sieves
- Iron oxide.
- Wood pellet impregnated with iron oxide.
- Iron oxide pellet.
- Membrane separation.
2.- Within the wet desulfurization processes .
- Wash with sodium hydroxide (NaOH). In one stage and in two stages.
- Washing with iron compounds
- Amine wash.
- biological desulfurization.
- Washing with pressurized water and low temperatures.
- Biochemical desulfurization.
- Desulfurization with a combination of techniques
In the gas desulfurization sector, as can be seen, there are numerous techniques, but there are two that predominate in the market when large flows are treated at high concentrations of H2 S. These are chemical washing, biological washing and biochemical washing, the latter, on many occasions with a stage of refining and drying
Each one of them is applied under specific conditions of flow rate, concentration of H 2 S and conditions of the current of the gas to be treated. However, when it comes to high concentrations of H 2 S, that is, above the> 5,000 ppm chemical or biochemical processes are the ones that report the best results.
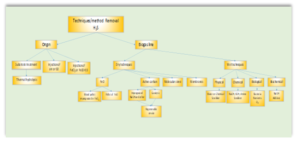
Figure 3 shows the different desulfurization processes applied to biogas cleaning according to the place where they are carried out and the technique/method used.
Comparison between processes.
Table Nº 1 presents the differences between the techniques used for gas desulfurization. It shows with +/- symbols the different characteristics of this type of process, regardless of the H 2 S load to be treated.
Table 1. Gas desulfurization process.
|
Elimination techniques. 1 2 3 4 5 6 7 |
| Dry.
Activated carbon – +/- +/- +/- + +/- +/- |
| Molecular sieves – +/- +/- +/- + +/- +/- Iron oxide pellet + + + +/- + +/- +/- Separación por membranas – – – – + +-Húmedos.Lavado NaOH +/- + +/- +/- + +/- +/- |
| Wash with iron solution +/- + +/- +/- + +/- +/- |
| Amine wash +/- + +/- +/- + +/- +/- |
| Biological washing – +/- +/- +/- + + +/- |
- Small-scale application (+ = yes) 2. Large-scale application (+ = yes)
- Simplicity (+ = simple) 4. Operation and maintenance (+ = low)
- H 2 S in the treated gas< 250 ppm (+ = yes) 6. Environmental impact (+ = low)
- Cost (+ = low).
In many cases, biological washing has lower operating costs than other technologies since it does not require the consumption of chemical products, but at the same time, it has important limitations that reduce its application among what can be mentioned.
- Applicable up to certain loads of H 2 S and flow of the gas to be treated. Generally below the< 5,000ppm H2S.
- It requires stability in the operating conditions and in the H2S concentration. In this process, it is living organisms that perform the degradation of H2S (bacteria). And like all living organisms, they require stable conditions to do the job.
- This type of process, when working with microorganisms, requires a certain time for their proliferation and startup, which is sometimes a disadvantage. This is because it requires a certain time for the development of the bacterial culture responsible for the process and its removal efficiency.
On the other hand, chemical washing, mainly with NaOH, is a well-known and applied technique. This technology is in continuous development to minimize reagent consumption, which is its main disadvantage, given the high concentration of CO2 present in the biogas, so a balance in the rates of reactions that take place is essential (NaOH + H2S and NaOH + H2S). This aspect is critical if good results in H2S removal efficiency and adequate operating costs are to be obtained. In these cases, it is usual to work with a solution with a pH between 8.6÷8.9
Studies have led to the appearance of combined processes that make it possible to recover, on the one hand, part of the NaOH used and, on the other hand, the sulfur in its elemental form S. Therefore, the combined techniques for the removal of H2S in biogas are the ones that best results are being reported so far for when they are treated, both high concentration of H2S in the biogas, and flows to be treated.
Figure 3 shows the biochemical scrubber with its different stages of operation: washing, oxidation and sedimentation that significantly minimizes the load of NaOH to be used and includes the refinement stage that guarantees stability of the process and concentrations of H2S in the biogas< 5ppm

Figure 2 . Biochemical scrubber + refinement stage

